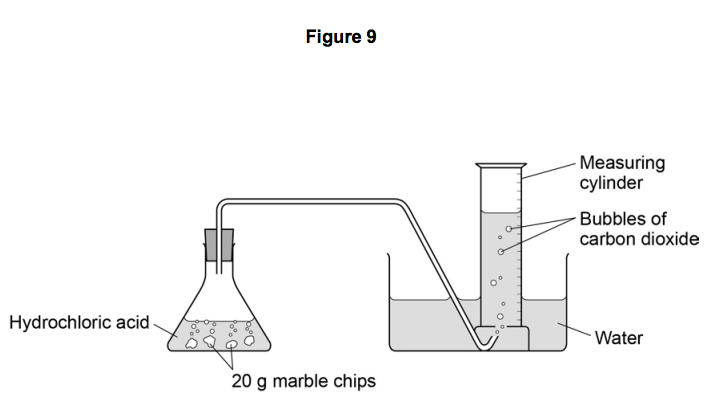
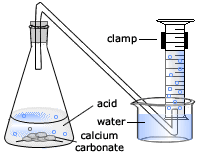
The rate of reaction between hydrochloric acid and marble chips plan in this experiment i will be investigating the rate of the reaction between hydrochloric acid hcl and marble chips.
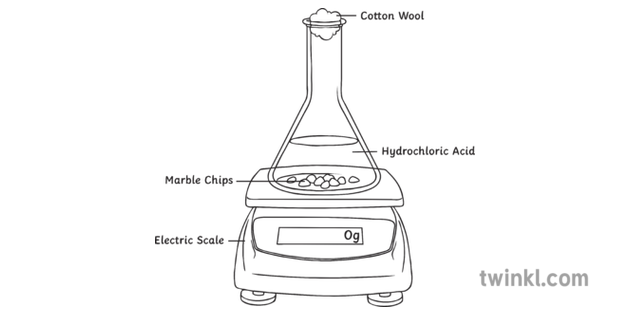
Marble chips and hydrochloric acid experiment equipment.
Hydrochloric acid 20ml 0 5m 1m 2m marble chips 2g per test large measuring cylinder plastic bowl 3 4 full of water rubber tubing glass conical flask stopwatch method.
Hydrochloric acid 20ml 0 5m 1m 2m marble chips 2g per test large measuring cylinder plastic bowl 3 4 full of water rubber tubing glass conical flask stopwatch method the first thing that we.
Diagram plan the equipment i will be using for this experiment will be as follows.
This report will investigate the influence of the concentration of reactants on the reaction rate by discussing an experiment which involves the reactants marble chips and hydrochloric acid.
Task my task is to measure the rate of reaction between marble chips caco 3 and hydrochloric acid 2 hcl.
Marble chips also known as calcium carbonate is a chemical compound with the molecular formula caco3.
Using a measuring cylinder add 50 cm 3 of dilute hydrochloric acid to a conical flask.
Marble chips calcium carbonate caco 3 react with hydrochloric acid hcl to produce carbon dioxide gas.
A chemistry investigation to look at the rates of reaction between marble chips and hydrochloric acid.
Conical flask delivery tube bung measuring cylinder x 2 water trough water stopwatch marble.
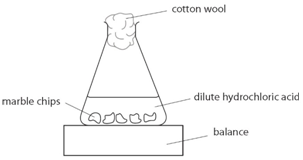
Add 0 4 g of calcium carbonate to the flask.
This experiment is to show how much carbon dioxide is produced during the reaction between an acid hydrochloric acid and marble.
The rate of this reaction can be changed by changing the size of the marble chips.
Calcium chloride solution is also formed.
An investigation of the reaction between marble chips and hydrochloric acid.
To do this i will change the concentration of the hcl and measure how that affects the amount of co2 produced during the reaction and hence find the rate.
Using the apparatus shown the change in mass of carbon dioxide can be measure with time.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
Immediately connect the gas syringe and start a stop clock.
This experiment is to show how much carbon dioxide is produced during the reaction between an acid hydrochloric acid and marble.